
St. Elmo’s fire, also known as St. Elmo’s light, is a strange weather phenomenon, in which, during thunderstorms a bright blue or violet glowing ball of light; fiery in appearance, is visible, under the low light conditions, from tall; sharply pointed structures such as lightning rods, masts on ships, spires on the top of buildings, especially skyscrapers and seen on church towers, chimneys, and on aircraft wings or aircraft nose cones, and on tall street lamps. The strange fiery phenomenon can also appear on leaves and grass and even at the tips of cattle horns. Often accompanying the glow is a distinct hissing or buzzing sound.
This fascinating burning torch like weather phenomenon is not ball lightning, but often times is mistaken for ball lightning. Ball lightning can float around the air, while St. Elmo’s fire is stationary.
Sailors, experiencing the St. Elmo’s fire on the masts of ship at sea during thunderstorms, have believed the awesome effect to be a good omen. The experience made the hearts of sailors filled with Christian religious admiration; reverence for their patron saint; the sailors believing they were in the presence of their blessed saint. St. Elmo’s fire is named after Saint Erasmus of Formica(also called St. Elmo), one of the two Italian names for St. Erasmus; the other being St. Erasmo, the Christian patron saint of sailors.
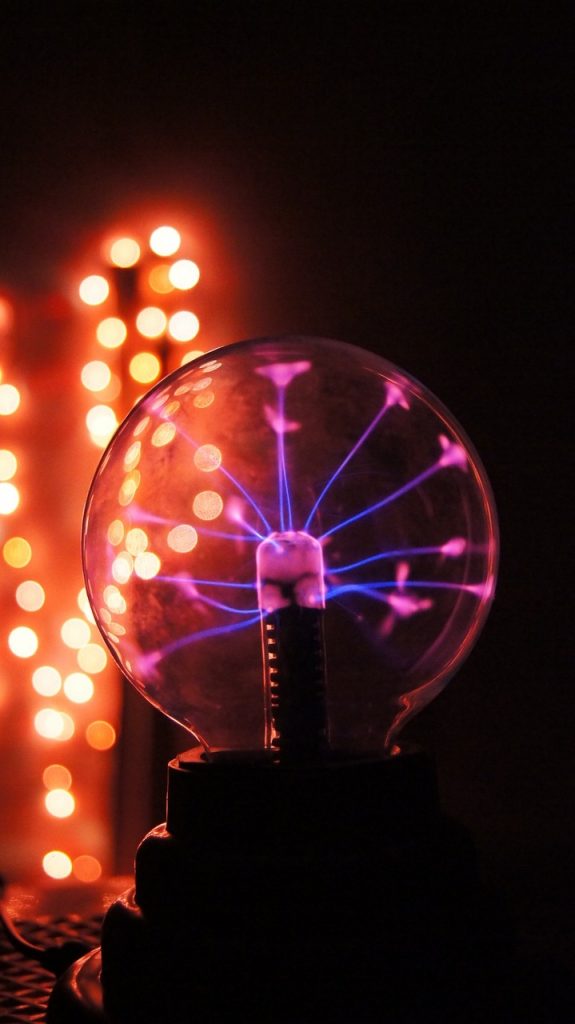
St. Elmo’s fire is produced when plasma-a component of stars, extreme temperature flame, and lightning has its electric field altered; ionized by atmospheric activity, around a certain object, especially a protruding, sharp object which can concentrate electrons; producing a corona discharge because there is a significant imbalance in the electric discharge, causing molecules to tear apart.
The thunderstorms release electrons in the air and the ground has a charge difference that creates voltage or electrical pressure in which the electrons move out; away from the protons on atoms. Nitrogen and oxygen in the atmosphere causes St. Elmo’s fire to fluoresce, like the system operating in glowing neon lights. Nitrogen and oxygen produce a blue glow in St. Elmo’s fire. Different gas in the air produce different colors with the St. Elmo’s fire.
The electrons are carried by the air and when the electrons accumulate enough, after being stored in a material electrical conductor object; they break out; pushing themselves into the air and burning in the gases that compose the air.
St. Elmo’s fire can be also produced during a volcanic eruption.
https://en.wikipedia.org/wiki/St._Elmo%27s_fire
http://science.howstuffworks.com/nature/climate-weather/atmosphere/st-emo-fire.html


